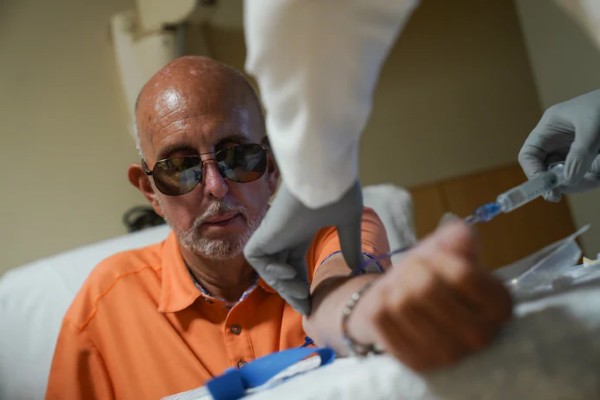
Jay Reinstein, who has early-stage Alzheimer’s, receives an injection in June for a PET scan at MedStar Georgetown University Hospital. The test is part of a clinical study, but the results could help him access Leqembi, a new drug that slows the disease. (Michael Robinson Chávez/The Washington Post)
From The Washington Post:
"Since being diagnosed five years ago with Alzheimer’s, Jay has felt desperate, sometimes panicky, about his fate. But now, he sees a sliver of hope: a new drug called Leqembi. It’s not a cure and does not restore memories destroyed by the fatal neurodegenerative disease. But the medication modestly slows progression of the condition, even as it raises significant safety and cost issues that are generating intense controversy.
The test Reinstein is undergoing on this day could help him get the drug. “I want more time to spend with my five grandchildren,” he said.
Leqembi, in a clinical trial, slowed cognitive decline by 27 percent over 18 months compared with a placebo. That represented a five-month delay in progression — dismissed as negligible by some but hailed as a milestone by others for a malady that has been largely untreatable. On Thursday, the Food and Drug Administration granted full approval to Leqembi — the first time such a clearance has been granted to a therapy that changes the course of the disease. Other Alzheimer’s drugs treat symptoms, and often not very well. The FDA action means Medicare, the federal health program for older Americans, will broadly cover the drug, according to Medicare officials."
Read more.
"Since being diagnosed five years ago with Alzheimer’s, Jay has felt desperate, sometimes panicky, about his fate. But now, he sees a sliver of hope: a new drug called Leqembi. It’s not a cure and does not restore memories destroyed by the fatal neurodegenerative disease. But the medication modestly slows progression of the condition, even as it raises significant safety and cost issues that are generating intense controversy.
The test Reinstein is undergoing on this day could help him get the drug. “I want more time to spend with my five grandchildren,” he said.
Leqembi, in a clinical trial, slowed cognitive decline by 27 percent over 18 months compared with a placebo. That represented a five-month delay in progression — dismissed as negligible by some but hailed as a milestone by others for a malady that has been largely untreatable. On Thursday, the Food and Drug Administration granted full approval to Leqembi — the first time such a clearance has been granted to a therapy that changes the course of the disease. Other Alzheimer’s drugs treat symptoms, and often not very well. The FDA action means Medicare, the federal health program for older Americans, will broadly cover the drug, according to Medicare officials."
Read more.

 RSS Feed
RSS Feed
