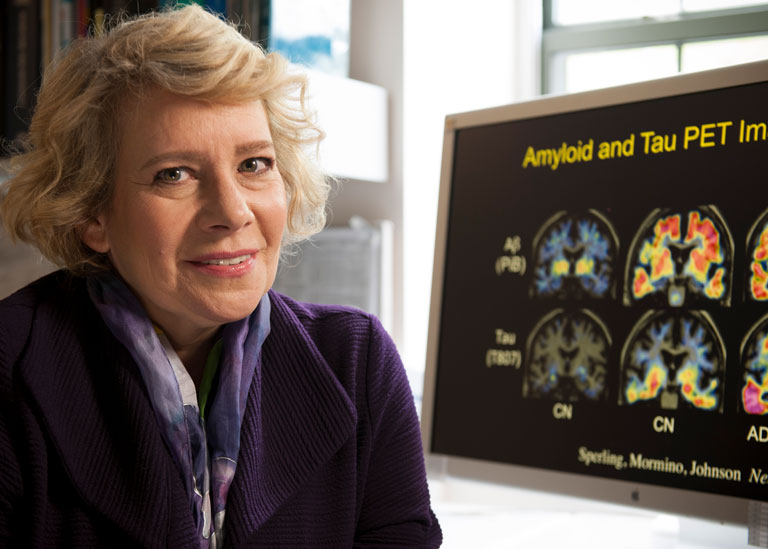
The article “Stopping Alzheimer’s Before Symptoms Appear” features pioneering work in prevention trials led by GHR partners Dr. Randall Bateman of Washington University in St. Louis, Dr. Reisa Sperling of Harvard University, and Dr. Eric Reiman of Banner Health. GHR CEO Amy Goldman highlighted the role that philanthropy can play in supporting trials. “Philanthropy …is willing to take risks and provide the seed money to get a trial started, and we can be patient with long-term, complex trials with a high potential for failure, especially with trials like these where there’s also high potential for impact.”
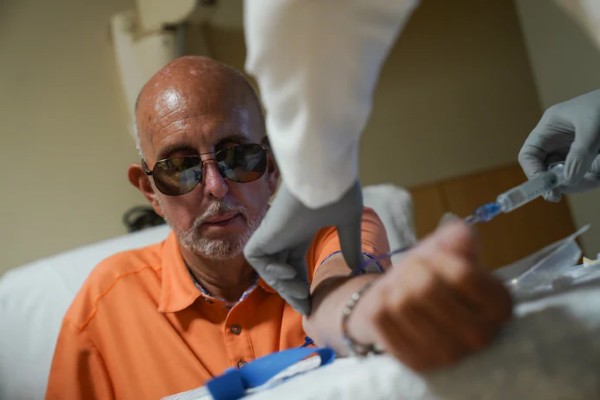
The article “Blood Tests for Alzheimer’s Could Become Routine” features GHR partners Dr. Randall Bateman and Dr. Nicolas Barthelemy at Washington University in St. Louis and their groundbreaking development of the first blood test for Alzheimer’s Disease.
Read the magazine here.





 RSS Feed
RSS Feed
