GHR Foundation’s Health initiative partners with organizations pursuing the prevention of Alzheimer’s disease, targeting funding to improve some of the largest research efforts in the field. One such partner is Washington University’s Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU). The DIAN-TU was formed to design and implement prevention trials for dominantly inherited Alzheimer’s disease, caused by a genetic mutation.
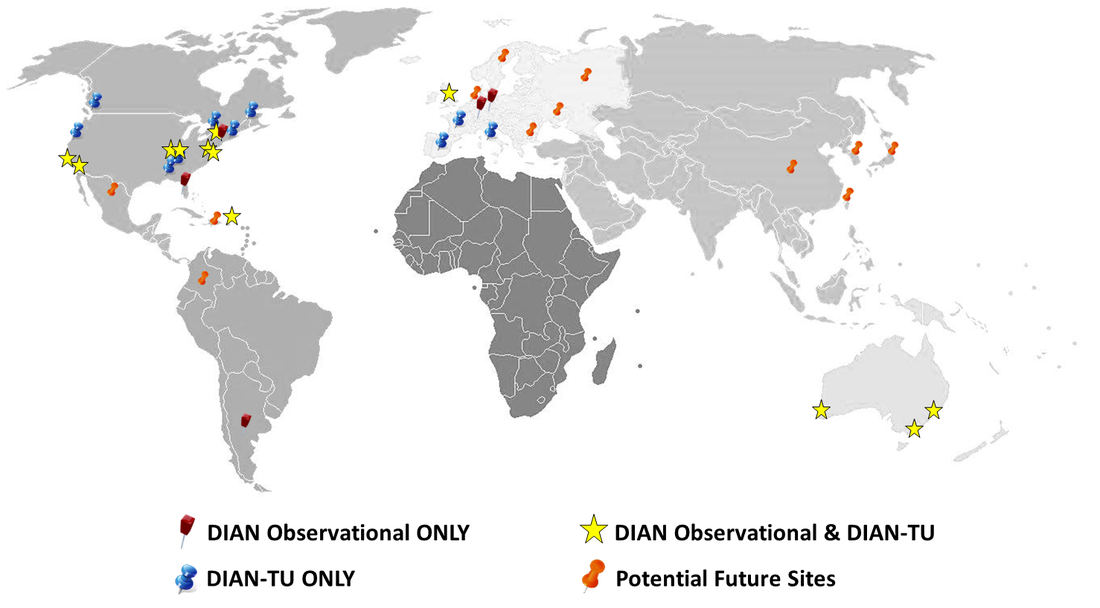
This rare form of Alzheimer’s disease results in early-onset of the disease with symptoms starting as early as a person’s 30s and 40s. The DIAN-TU launched a prevention study in this rare population testing experimental drug therapies that may stop the progression of Alzheimer’s disease before symptoms emerge. The trial is currently fully enrolled for the first two drugs and operational in 24 sites across six countries, with interim results expected in 2017 and final results by 2019.
The DIAN-TU builds on Washington University's Dominantly Inherited Alzheimer Network Observational study which collects observational data on persons with these genetic mutations. The DIAN study is operational in 17 sites across six countries, many of which are also DIAN-TU trial sites.
Washington University is currently planning its DIAN-TU Next Generation trials, which will test two additional potential treatments and novel diagnostic approaches with the dominantly inherited Alzheimer’s disease population. The DIAN-TU project gained attention recently in Germany during the first meeting of German families with the mutations. Attendees were informed about Alzheimer’s clinical trials and the DIAN-TU trials in particular, and had the opportunity to pose questions to researchers. All eligible attendees indicated interest in enrolling in future studies. Given the requirement for multiple sites to meet recruitment goals in this rare population, the participation of the German families is an important step for the DIAN-TU Next Generation Trials.
To learn more about how GHR is joining forces with industry, other philanthropic partners and the United States National Institutes of Health on the prevention of Alzheimer’s disease, contact us. To learn more about the DIAN-TU Study, visit www.dianexr.org or call 1-844-DIAN-EXR (844-342-6397).
This rare form of Alzheimer’s disease results in early-onset of the disease with symptoms starting as early as a person’s 30s and 40s. The DIAN-TU launched a prevention study in this rare population testing experimental drug therapies that may stop the progression of Alzheimer’s disease before symptoms emerge. The trial is currently fully enrolled for the first two drugs and operational in 24 sites across six countries, with interim results expected in 2017 and final results by 2019.
The DIAN-TU builds on Washington University's Dominantly Inherited Alzheimer Network Observational study which collects observational data on persons with these genetic mutations. The DIAN study is operational in 17 sites across six countries, many of which are also DIAN-TU trial sites.
Washington University is currently planning its DIAN-TU Next Generation trials, which will test two additional potential treatments and novel diagnostic approaches with the dominantly inherited Alzheimer’s disease population. The DIAN-TU project gained attention recently in Germany during the first meeting of German families with the mutations. Attendees were informed about Alzheimer’s clinical trials and the DIAN-TU trials in particular, and had the opportunity to pose questions to researchers. All eligible attendees indicated interest in enrolling in future studies. Given the requirement for multiple sites to meet recruitment goals in this rare population, the participation of the German families is an important step for the DIAN-TU Next Generation Trials.
To learn more about how GHR is joining forces with industry, other philanthropic partners and the United States National Institutes of Health on the prevention of Alzheimer’s disease, contact us. To learn more about the DIAN-TU Study, visit www.dianexr.org or call 1-844-DIAN-EXR (844-342-6397).

 RSS Feed
RSS Feed
